
Modern periodic table with atomic numbers listed above atom symbols. If you look at a periodic table, you will notice that each element has a unique value between 1 and 118 which chemists call “atomic number.” Hydrogen has an atomic number of 1. What exactly makes one element different from another? Why are carbon and hydrogen and oxygen considered to be different substances? What can we specifically point to that explains the difference between these elements on its most basic level? Geological specimens are known in which the element has an isotopic composition outside the limits for normal material.In this article, you will learn about atomic number, its definition, its usefulness in categorizing elements, and its history as a theory in chemistry.See table 1 for details of range and original paper for the atomic weight of the element from different sources.However three such elements (Th, Pa, and U) do have a characteristic terrestrial isotopic composition, and for these an atomic weight is tabulated. , indicates the mass number of the longest-lived isotope of the element. Substantial deviations in atomic weight of the element from that given in the Table can occur. Modified isotopic compositions may be found in commercially available material because it has been subject to an undisclosed or inadvertant isotopic fractionation.Value being given the tabulated value should be applicable to any normal material. Range in isotopic composition of normal terrestrial material prevents a more precise.The difference between the atomic weight of the element in such specimens and that given in the Table may exceed the stated uncertainty. Geological specimens are known in which the element has an isotopic composition outside the limits for normal material.See original paper for the range of these elements from different sources List of Elements with Range of Atomic Weights. See also a copy of the periodic table with atomic weights to five significant figures. See below for the elements listed in Atomic Number Order or Name order.
#Periodic table hydrogen atomic number full
The original paper should be consulted for full details of the variation in atomic weight and the half life of the radioisotopes quoted below.Ī number in parentheses indicates the uncertainty in the last digit of the atomic weight.
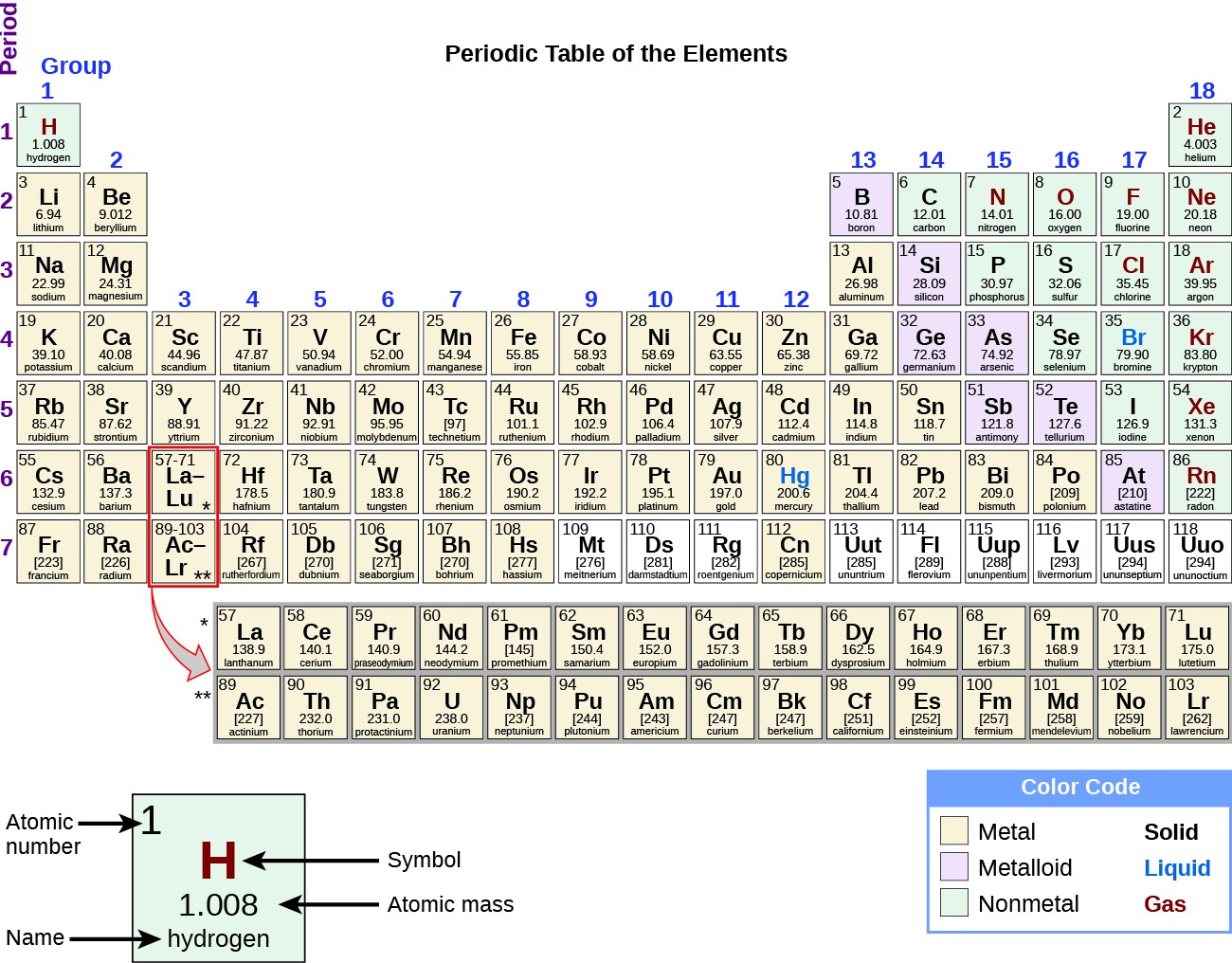

For radioactive elements the isotope with the longest half-life is quoted in parenthesis. In the other lists the values quoted are those suggested for material where the origin of the sample is unknown. The standard atomic weights of twelve elements having two or more stable isotopes have variability of atomic-weight values in natural terrestrial materials. Previous values may be consulted from the 1993 table, the 1995 table, the 1997 table, the 1999 table, the 2001 table, the 2005 table, the 2007 table, the 2009 table, the 2011 table, the 2013 table, the 2015 table or the 2019 table. World Wide Web version of atomic weight data originally prepared by G.
:max_bytes(150000):strip_icc()/hydrogen-on-the-periodic-table-113718224-5810edd83df78c2c73139e47.jpg)
These tables are based on the 2021 table with changes from the 2019 table for the values of Ar, Hf, Ir, Pb and Yb and changes to the uncertainty for Al, Au, Co, F, Ho, Mn, Nb, Pa, Pr, Rh, Sc, Tb, Tm, and Y. 2021 Atomic Weights IUPAC Commission on Isotopic Abundances and Atomic Weights.


 0 kommentar(er)
0 kommentar(er)
